The five tandem projects study how molecular adjustments tune specific signalling pathways of neuronal communication.
Prof. Robert Fledrich, Institute of Anatomy, Medical Faculty, Leipzig University
Prof. Ines Liebscher, Rudolf Schönheimer Institute of Biochemistry, Medical Faculty, Leipzig University
Project Description
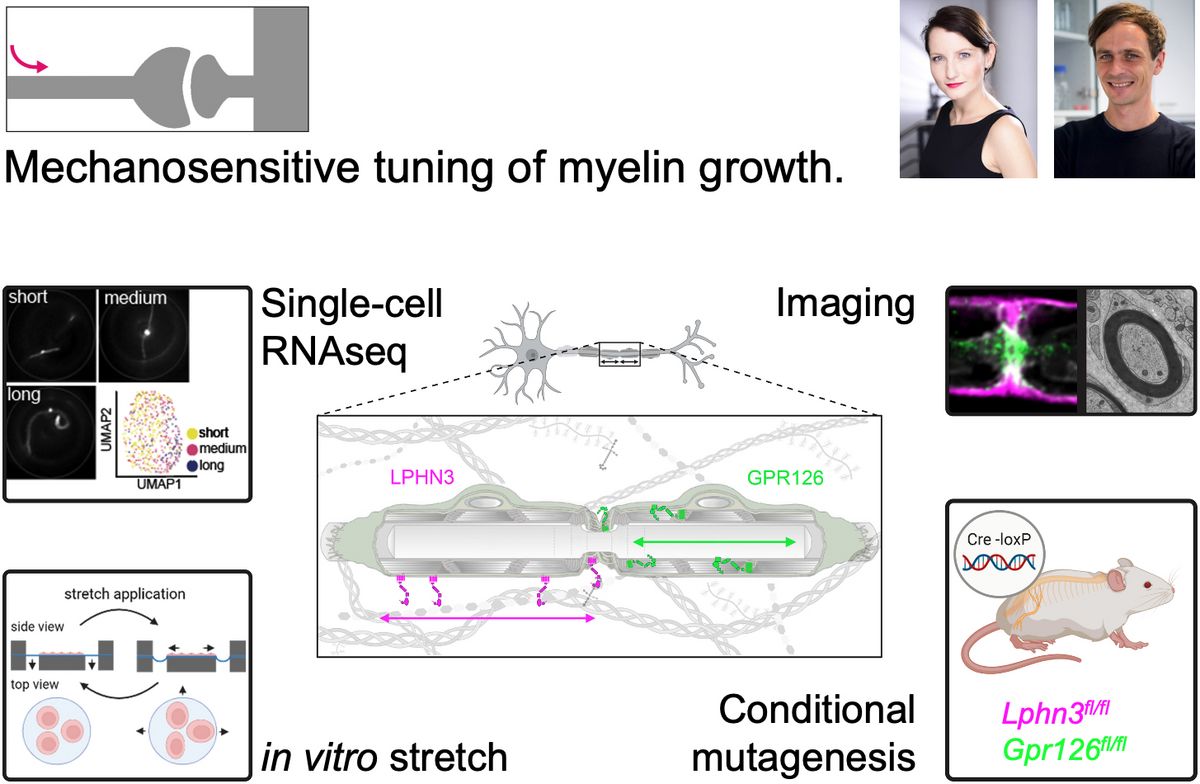
Myelin is an insulating sheath surrounding axons, essential for rapid and efficient signal transmission, and is frequently disrupted in neurological disorders such as multiple sclerosis and peripheral neuropathies. Myelination of axons occurs in a segmental pattern, with glial cells forming myelin sheaths of up to 2 mm in length, interspersed with nodes of Ranvier as sites for action potential generation. These internodal myelin segments provide the anatomical foundation for saltatory conduction, a process that underpins fast neuronal impulse propagation. The length of the myelin segments profoundly influences conduction velocity, yet the mechanisms that regulate longitudinal myelin growth and determine the final internodal length (INL) are not known.
In this project, we investigate how external mechanical cues, including axonal tension and extracellular matrix signals, are transduced into intracellular biochemical pathways in myelinating glia to modulate longitudinal myelin growth. We focus particularly on adhesion G protein-coupled receptors (aGPCRs) such as GPR126, which are recognized for their role in integrating mechanical signals. By elucidating how these receptors contribute to the regulation of myelin elongation and axonal conduction, we aim to offer new insights into myelin biology and potential avenues for treating demyelinating diseases.
Host Lab
The Fledrich lab investigates axon-glia interactions in health and disease with special emphasis on myelin as the anatomical prerequisite of fast axonal conduction. We offer transgenic techniques in mice, a battery of state of the art behavioral and electrophysiological methods, confocal imaging, 3D electronmicroscopy and single cell sequencing approaches, to unravel mechanisms that may help to identify therapeutic targets for life-burdening diseases such as Multiple sclerosis.
The Liebscher Lab investigates the activation mechanism and physiological implications of a class of orphan G protein-coupled receptor (GPCR). These adhesion GPCRs are sensors of the cellular context and respond among others to extracellular matrix molecules and mechanical forces. We employ a set of second messenger detection systems, 2D and 3D matrix cell cultures, stretch devices and mouse models.
Requirements
The candidates should have a profound background in molecular biology, ideally with prior experience in one or more of the following areas: histology, protein biochemistry, genetics, fluorescence microscopy, electron microscopy, behavioural analysis with mice.
Selected publications
Fledrich R, Abdelaal T, Rasch L., … , Bechmann I, Nave KA, Stassart RM, Sereda MW (2018) Targeting myelin lipid metabolism as a potential therapeutic strategy in a model of CMT1A neuropathy. Nature Communications 9(1):3025. doi: 10.1038/s41467-018-05420-0.
Ping YQ, Xiao P, Yang F, Zhao RJ, Guo SC, Yan X, Wu X, Zhang C, Lu Y, Zhao F, Zhou F, Xi YT, Yin W, Liu FZ, He DF, Zhang DL, Zhu ZL, Jiang Y, Du L, Feng SQ, Schöneberg T, Liebscher I*, Xu HE*, Sun JP* (2022) Structural basis for the tethered peptide activation of adhesion GPCRs. Nature 604(7907):763-770. doi: 10.1038/s41586-022-04619-y.
Dr. Kristina Lippmann, Carl-Ludwig-Institute for Physiology, Medical Faculty, Leipzig University
Prof. Dr. Ruth Stassart, Paul Flechsig Institute – Centre of Neuropathology and Brain Research, Medical Faculty, Leipzig University
Project description
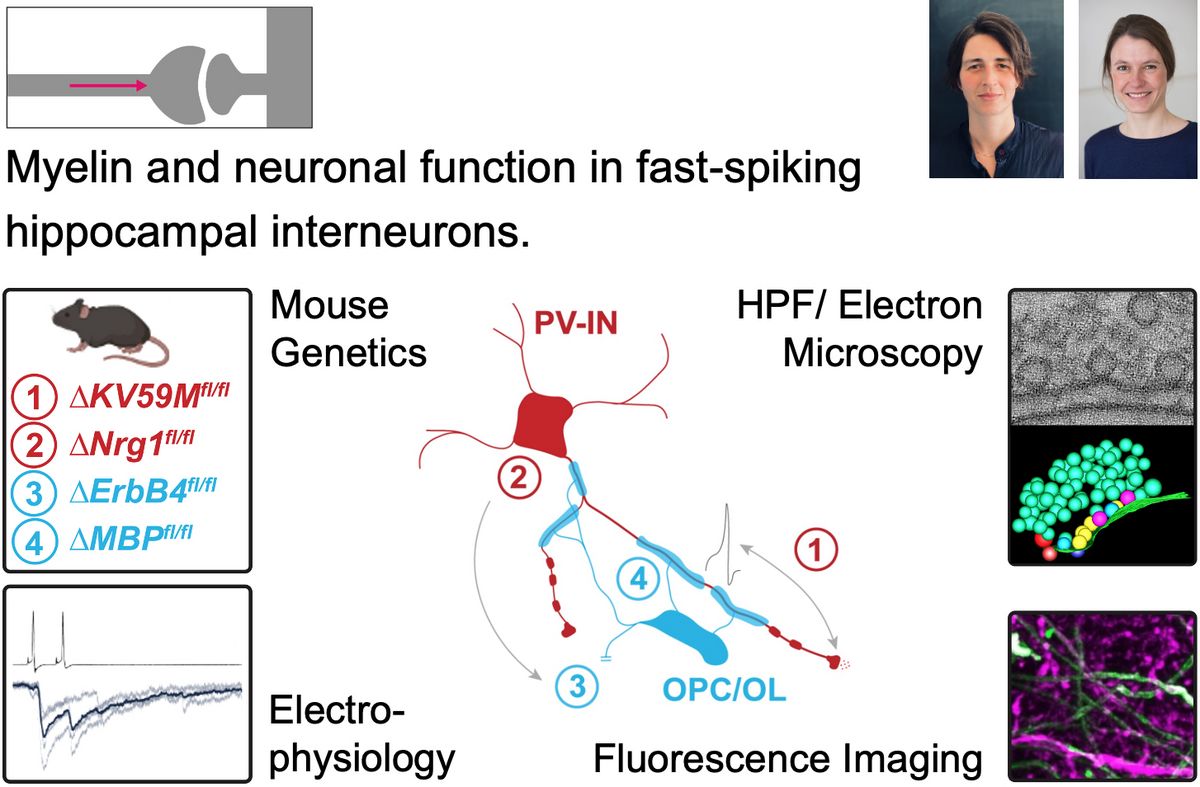
Parvalbumin-expressing GABAergic interneurons (PV-INs) provide robust inhibition in neural networks. They are essential for gamma oscillations and sharp-wave ripple generation, which are critical for cognitive functions such as learning, memory, and information replay. PV-INs are closely associated with oligodendroglia and exhibit a patchy myelination pattern along their axonal arbors. Yet, the role of myelin in regulating PV-IN maturation and plasticity is not well defined. Disruption of PV-IN function has been observed in various neurological disorders, including multiple sclerosis, leukodystrophies and epilepsy; however, the underlying pathomechanisms remain poorly understood. We will investigate the unclear interdependence between PV-IN myelination and intrinsic-to-synaptic function, as well as their impact on cognition-associated network oscillations. We will utilize conditional mouse models that affect either myelination or presynaptic release to explore these fundamental research questions. Our methodology will involve advanced techniques such as electrophysiology, light sheet-, confocal- and two-photon microscopy, electron tomography, transcriptomics, and behavioral analysis, aiming to gain mechanistic insight into regulatory processes that coordinate PV-IN axo-synaptic activity, synaptic network integration, and (adaptive) myelination. These studies will significantly enhance our understanding of PV-IN functions in both healthy and diseased brains and aid in developing more effective treatment strategies for epilepsy and myelin disorders.
Host labs
Kristina Lippmann’s research group integrates cellular-to-network electrophysiology and high-pressure freezing with electron tomography to investigate the functional and ultrastructural mechanisms of presynaptic release and plasticity. In this context, the lab further examines how acquired and genetic models of cognition- and epilepsy-related disorders impact the presynaptic function and structure of interneurons and their associated network oscillations.
The lab of Ruth Stassart studies fundamental principles of axon-glia signaling and how disruptions in this interplay lead to myelin disorders, such as leukodystrophies and multiple sclerosis. To address these questions, the lab employs a wide range of techniques, including conditional mouse mutagenesis, behavioral phenotyping, advanced light and (3D)-electron microscopy, molecular biology, single-cell and spatial transcriptomics, as well as human neuropathology.
Requirements
The ideal candidates should have prior experience in at least one of the following fields: working with mice, patch-clamp electrophysiology, network physiology, behavioral analysis, fluorescence microscopy, two-photon microscopy, (cryo-) electron tomography, immunohistochemistry, or molecular biology.
Selected publications
Burkart ME*, Kurzke J*, Jacobi R, Vera J, Ashcroft FM, Eilers JE, Lippmann K (2024)
KATP channel mutation disrupts hippocampal network activity and nocturnal gamma shifts. Brain awae157.
Schäffner E, Bosch-Queralt M, Edgar JM, Lehning M, Strauß J, Fleischer N, Kungl T, Wieghofer P, Berghoff SA, Reinert T, Krueger M, Morawski M, Möbius W, Barrantes-Freer A, Stieler J, Sun T, Saher G, Schwab MH, Wrede C, Frosch M, Prinz M, Reich DS, Flügel A, Stadelmann C, Fledrich R, Nave KA, Stassart RM. (2023) Myelin insulation as a risk factor for axonal degeneration in autoimmune demyelinating disease. Nature Neuroscience 26:1218-1228.
Prof. Robert J. Kittel, Department of Animal Physiology, Institute of Biology, Faculty of Life Sciences, Leipzig University
Prof. Stefan Hallermann, Carl-Ludwig-Institute of Physiology, Medical Faculty, Leipzig University
Project description
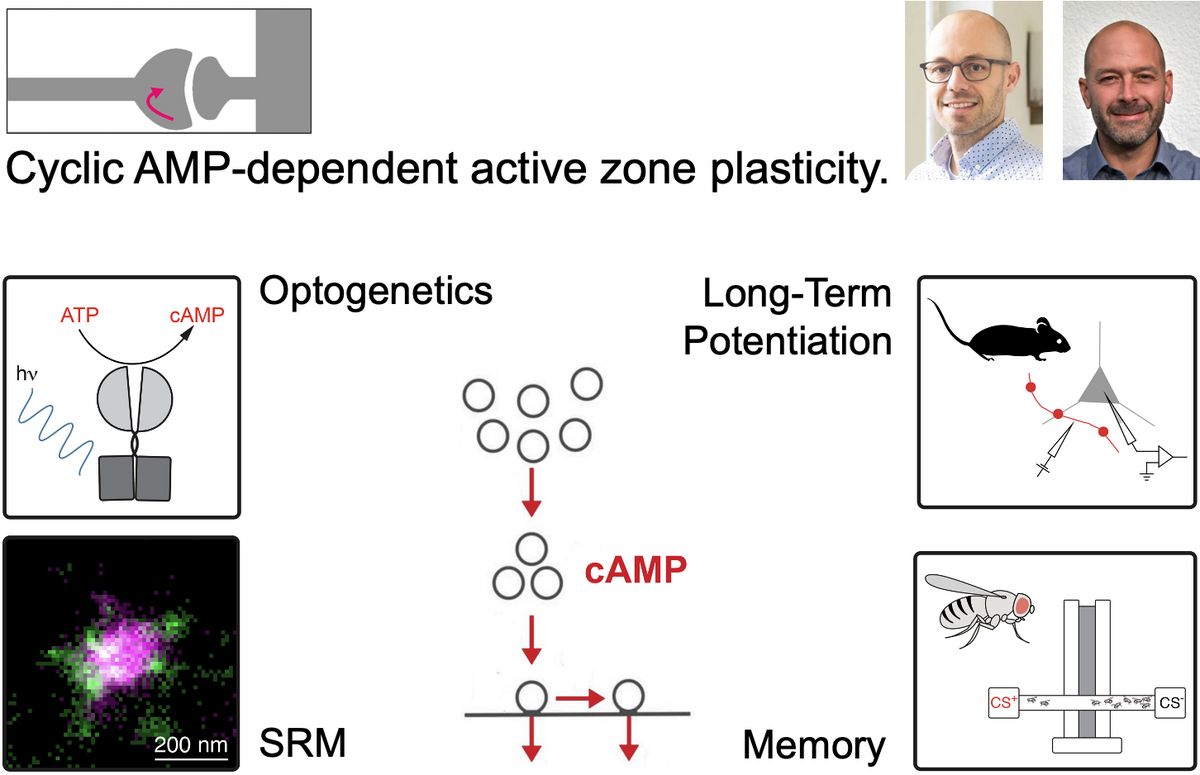
Memories define us as individuals by linking our past with our present. The capacity to learn is fundamental to animal survival and has thus been the subject of intensive scientific research. Numerous studies in vertebrate and invertebrate systems have revealed the pivotal role of synaptic plasticity in learning and memory, but despite decades of work, fundamental questions regarding the underlying molecular mechanisms remain unanswered. This is partly due to major methodological challenges for studying neurotransmission in vivo as well as difficulties to analyse the structure and function of synapses with high spatial and temporal resolution. This project will focus on cyclic AMP-dependent modifications at the presynaptic site of neurotransmitter release – the active zone. We will combine experiments in Drosophila and mouse, in vitro and in vivo, and at sub-cellular and behavioural levels to improve our mechanistic understanding of the plastic molecular processes underlying learning and memory.
Host labs
With the Hallermann lab focussing primarily on rodents and the Kittel lab on Drosophila, we have established a variety of methods ideally suited to investigate active zone plasticity with high spatial and temporal resolution. These include optical and optogenetic tools to measure and to manipulate intracellular cyclic AMP levels in vitro and in vivo, electrophysiological recordings from presynaptic terminals, 2-photon calcium-uncaging and -imaging techniques, computational modelling, behavioural readouts, and super resolution microscopy (SRM) of the active zone.
Requirements
The ideal candidates should have prior experience in at least one of the following methods: electrophysiology, live-cell imaging, immunohistochemistry, molecular biology, or behavioural readouts. Familiarity with Drosophila melanogaster, rodent experiments, or cell culture work is preferred.
Selected publications
Bullmann T*, Kaas T*, Ritzau-Jost A*, Wöhner A, Kirmann T, Rizalar FS, Holzer M, Nerlich J, Puchkov D, Geis C, Eilers J, Kittel RJ, Arendt T, Haucke V, Hallermann S (2024) Human iPSC-derived neurons with reliable synapses and large presynaptic action potentials. J Neurosci 44:e0971232024. doi: 10.1523/JNEUROSCI.0971-23.2024.
Sachidanandan D*, Aravamudhan A*, Mrestani A, Nerlich J, Lamberty M, Hasenauer N, Ehmann N, Pauls D, Seubert T, Maiellaro I, Selcho M, Heckmann M, Hallermann S#, Kittel RJ# (2023) Rab3 mediates cyclic AMP-dependent presynaptic plasticity and olfactory learning. bioRxiv doi: 10.1101/2023.12.21.572589.
Prof. Tobias Langenhan, Rudolf Schönheimer Institute of Biochemistry, Medical Faculty, Leipzig University & Institute of Biology, Faculty of Life Sciences, Leipzig University
Prof. Andreas Thum, Institute of Biology, Faculty of Life Sciences, Leipzig University
Project description
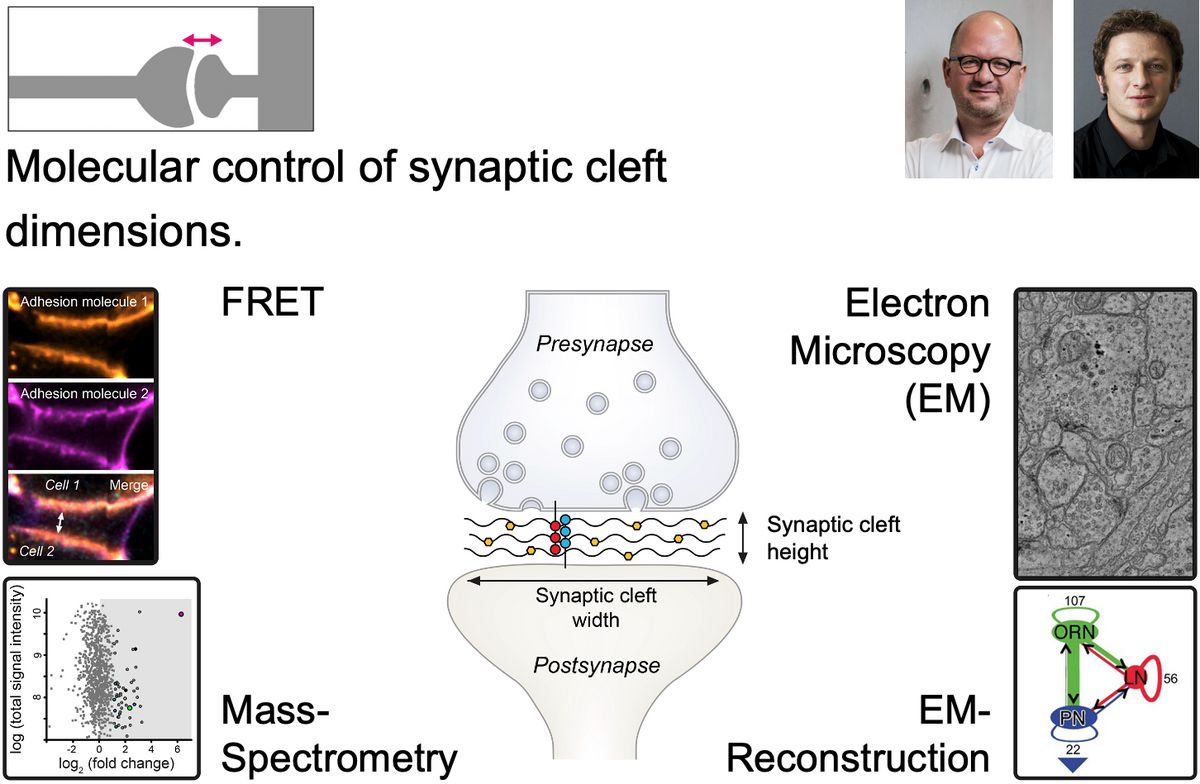
The synaptic cleft is the compartment that divides but also connects the pre- and postsynapse of neurons. It is a largely ignored structural component of synapses, which, however, must bear significant impact on chemical neurotransmission due to its position and size. The molecular contents of the synaptic cleft are only rudimentarily catalogued, the regulation of its dimensions including width and area are not understood, and the impact of the former two on synaptic communication are unknown.
In project 4 the successul doctoral applicants will break new scientific ground in the Langenhan and Thum labs and develop molecular tools to study the components of two example synapses with different morphological properties, glutamatergic and cholinergic contacts, in the fruit fly Drosophila melanogaster. The candidates will engineer and transgenically express baits for synaptic cleft expression to fish for synaptic cleft components, identify them via mass-spectrometric analysis, compare them between the synapse types and anatomically quantity their impact on synaptic cleft size and structure. The candidates will investigate the functional role of selected synaptic cleft molecules found in the biochemical screens through genetic, behavioral and anatomical analyses to understand how they contribute to synaptic transmission.
Host labs
The Langenhan lab investigates mechanisms of synaptic transmission in the context of molecular pathways that are controlled through adhesive and mechanical stimuli with emphasis on adhesion GPCRs, adhesion complexes and intracellular multi-molecular machines. A wide range of in-house techniques are available including: Drosophila as a model organism, molecular cloning, genomic engineering, different localisation and functional imaging techniques (confocal, FRET, dSTORM), electrophysiology, protein biochemistry, behaviour assays, bioinformatics.
The Thum lab explores the mechanisms of synaptic transmission and its plasticity, with a particular focus on chemosensation and learning and memory. The lab employs a variety of techniques, including the use of Drosophila as a model organism, high-resolution light microscopy, electron microscopy, connectomics, and an extensive set of behavioural methods to study both naïve and learned behaviours.
Requirements
The ideal candidates should have prior experience in at least one of the following areas: working with Drosophila melanogaster, protein biochemistry, mass spectrometry, immunohistochemistry, behavioural analysis, connectomics, or electron microscopy. Familiarity with molecular biology techniques, confocal or super-resolution imaging, or genetics is preferred but no must.
Selected publications
Scholz N*,#, Dahse A-K*, Kemkemer M, Bormann A, Auger GM, Contreras FV, Ernst LF, Staake H, Körner MB, Buhlan M, Meyer-Mölck A, Chung YK, Blanco-Redondo B, Klose F, Jarboui MA, Ljaschenko D, Bigl M, Langenhan T# (2023) Molecular sensing of mechano- and ligand-dependent adhesion GPCR dissociation. Nature 615:945–953.
Eichler K, Li F, Litwin-Kumar A, Park Y, Andrade I, Schneider-Mizell CM, Saumweber T, Huser A, Eschbach C, Gerber B, Fetter RD, Truman JW, Priebe CE, Abbott LF, Thum AS, Zlatic M, Cardona A. (2017) The complete connectome of a learning and memory centre in an insect brain. Nature 548:175-182.
PD Dr. Mareike Selcho, Institute of Biology, Faculty of Life Sciences, Leipzig University
Dr. Christian M. Simon, Carl-Ludwig-Institute of Physiology, Medical Faculty, Leipzig University
Project description
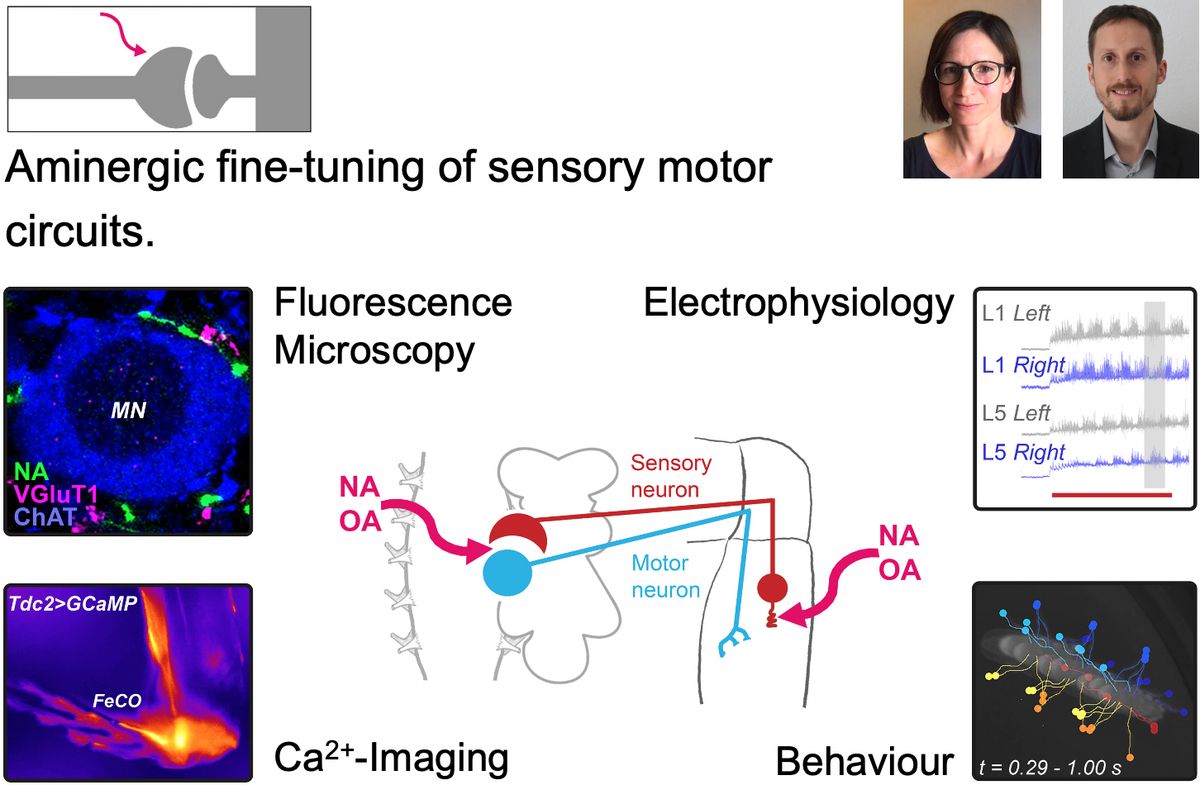
The spinal sensory-motor circuit is conserved in almost all motile organisms, facilitating precise voluntary movements and complex motor coordination. The aminergic neuromodulator noradrenaline (NA) in vertebrates and its functional analog octopamine (OA) in invertebrates crucially fine-tune the function of sensory-motor circuits in health and neurodegeneration. However, the mechanisms through which NA and OA modulate each cellular component of the neuronal network remain largely unknown. Therefore, we will investigate how NA/OA fine-tunes somatosensors and motor neurons in both health and disease, and explore the release machinery to gain a deeper understanding of the molecular composition of aminergic release sites.
Host labs
The Selcho lab is interested in the modulatory mechanisms that enable behavioural plasticity. We are working on the anatomical and functional characterization of aminergic neurons in the brain and periphery of Drosophila using light microscopy and EM, connectomics, Ca2+- and cAMP-Imaging, and behavioural analyses.
The Simon lab focusses on understanding the synaptic and cellular mechanisms involved in neurodegenerative diseases. We are studying morphology and function of the sensory-motor circuit in different disease mouse models using patch clamp recordings in the intact spinal cord, confocal and super resolution microscopy (SRM), viral gene manipulation, and behavioural assays.
Requirements
We are looking for new highly motivated group members interested in investigating the role of NA/OA in fine-tuning of the sensory-motor circuit. Prior experience in the following areas is beneficial but not mandatory: working with Drosophila or mice, microscopy techniques (incl. SRM), behavioural analysis, Ca2+- and cAMP-Imaging, electrophysiology, and neurobiology.
Selected publications
Dorkenwald S, Matsliah A, Sterling AR, Schlegel P, Yu SC, McKellar CE, Lin A, Costa M, Eichler K, Yin Y, Silversmith W, Schneider-Mizell C, Jordan CS, Brittain D, Halageri A, Kuehner K, Ogedengbe O, Morey R, Gager J, Kruk K, Perlman E, Yang R, Deutsch D, Bland D, Sorek M, Lu R, Macrina T, Lee K, Bae JA, Mu S, Nehoran B, Mitchell E, Popovych S, Wu J, Jia Z, Castro MA, Kemnitz N, Ih D, Bates AS, Eckstein N, Funke J, Collman F, Bock DD, Jefferis GSXE, Seung HS, Murthy M, FlyWire Consortium (Selcho M) (2024) Neuronal wiring diagram of an adult brain. Nature 634:124-138. doi: 10.1038/s41586-024-07558-y.
Simon CM, Delestrée N, Montes J, Sowoidnich L, Gerstner F, Carranza E, Buettner JM, Pagiazitis JG, Prat-Ortega G, Ensel S, Donadio S, Dreilich V, Carlini MJ, Garcia JL, Kratimenos P, Chung WK, Sumner CJ, Weimer LH, Pirondini E, Capogrosso M, Pellizzoni L, De Vivo DC, Mentis GZ (2025) Proprioceptive synaptic dysfunction is a key feature in mice and humans with spinal muscular atrophy. Brain awaf074. doi: doi.org/10.1093/brain/awaf074.